 Loading... Please wait...
Loading... Please wait...- Home
- Trucking Directories
-
Truckers News Feed
- Semi Truck Accidents News Reports
- Bus Accidents News Reports
- Current USA Diesel Fuel Prices
- Take Our Border Back Convoy News Coverage Live Streams Schedule Route Activities
- USA Real Time Road Conditions
- FMCSA DOT CDL News Regulations Enforcement Actions
- Trucking Companies Driver Scams Ripoffs Hiring Training Leasing
-
Video Library
- EXTREME Big Rig Truck Wrecks Crashes Accidents Videos
- Custom Big Rig Semi Truck Videos
- Tutorial Video National Registry Medical Examiner
- Big Rig Trucker Training Videos
- Heavy Haul Trucking Big Rig Videos
- FasterTrucks Road Train Videos
- Perils of Speed Limiters in Big Rig Trucks Videos
- Global Truckers Videos
-
Articles
- Top 50 Tips Every Truck Driver Should Know
- Truck Driver Safe Driving Rules
- Downsides of Driving a Truck in Winter
- How to Find a Truck Driving School
- Top 5 Characteristics Successful Truck Drivers
- Truck Driver Accident Procedures
- Owner Operator Tax Deductions
- DOT CDL Commercial Vehicle Inspection Procedures
- HOS New Hours of Service Rules FAQ Truckers
- SafetyPass Pro
- Search Trucking
- Contact Us
CDL Drivers DOT Medical Exam Form MCSA-5875 PDF
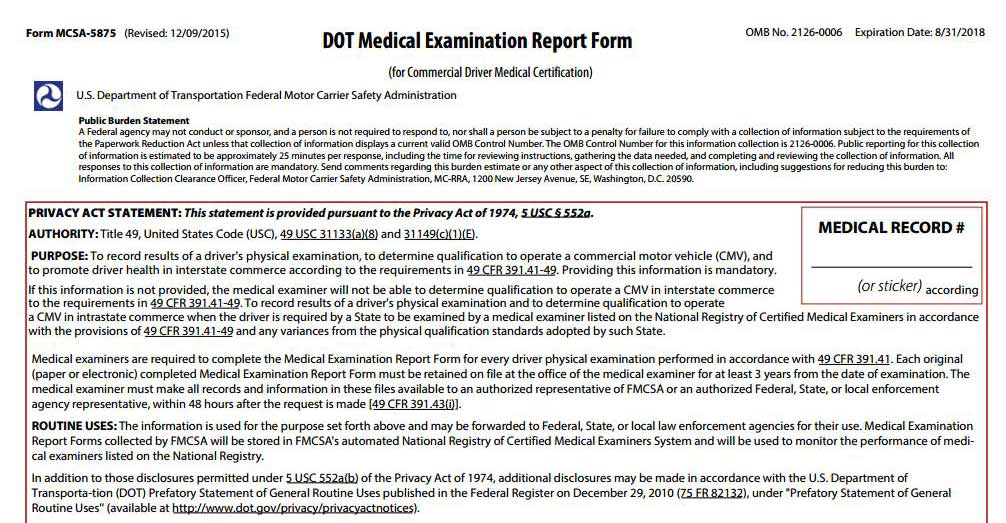
Current Form & Medical Card
Free to Print or Download.Videos How To Chain Up Big Trucks Tire Chains Instructions

Videos - Learn How To Chain Up a Big Rig Semi Truck or 18 Wheeler. Experienced Truck Drivers Show You How to do it. Single Tire Chains and Double Tire Chains (Three-Railers) Installation Instructions.
Truck Driver Safe Driving Rules

Best Safe Driving Tips for Truck Drivers. Watch Videos, See what happens when safe driving rules are violated.
CDL Drivers Medical Examination Requirements

CDL Drivers Medical Minimum Requirements
CDL Drivers Medical Examination Physicians Instructions

DOT CDL Medical Examiner's Instructions
Big Rig Trucker Training Videos

Learn how to shift 10-speed through 18-speed manual transmissions. See How to backup into narrow docks, How to handle truck tire blowouts and how to drive in traffic.
- Home
- Trucking Directories
- DOT CDL Physical Exam Locations and Forms USA
- DOT Drug Testing Form CCF OMB 0930 0158 pdf
Get Your DOT Physical Exam & CDL Medical Card Fast! Use this directory to check for the lowest cost & locate Active, Certified (CME) physicians near you. Concentra Medical locations included. Choose your doctor & Call for Appointment.
DOT Drug Testing Form CCF OMB 0930 0158 pdf
Updated 9-9-2020. Federal DOT Drug Testing Custody Control Form (CCF) OMB-0930-0158 for transportation industry DOT physical examinations and "Safety-Sensitive" employees. PDF-Printable-Free. Includes Instructions to Specimen Collectors and Maximum Allowable Drug Analyte Concentrations. Get Adobe Reader Here
Instructions for Completing the Federal Drug Testing Custody and Control Form (CCF).
When making entries use black or blue ink pen and press firmly Collector ensures that the name and address of the HHS-certified Instrumented Initial Test Facility (IITF) or HHS-certified laboratory are on the top of the CCF and that the Specimen I.D. number on the top of the CCF matches the Specimen I.D. number on the labels/seals.
STEP 1:
Collector ensures that the required information is in STEP 1. Collector enters a remark in STEP 2 if Donor refuses to provide his/her SSN or Employee I.D. number.
Collector gives collection container to Donor and instructs Donor to provide a specimen. Collector notes any unusual behavior or appearance of Donor in the remarks line in STEP 2. If Donor conduct at any time during the collection process clearly indicates an attempt to tamper with the specimen, Collector notes the conduct in the remarks line in STEP 2 and takes action as required.
STEP 2:
Collector checks specimen temperature within 4 minutes after receiving the specimen from Donor, and marks the appropriate temperature box in STEP 2. If temperature is outside the acceptable range, Collector enters a remark in STEP 2 and takes action as required.
Collector inspects the specimen and notes any unusual findings in the remarks line in STEP 2 and takes action as required. Any specimen with unusual physical characteristics (e.g. unusual color, presence of foreign objects or material, unusual odor) cannot be sent to an Instrumented Initial Test Facility (IITF) and must be sent to a Department of Health and Human Services (HHS) certified laboratory for testing as required.
Collector determines the volume of specimen in the collection container. If the volume is acceptable, Collector proceeds with the collection. If the volume is less than required by the Federal Agency, Collector takes action as required, and enters remarks in Step 2. If no specimen is collected by the end of the collection process, Collector checks the None Provided box, enters a remark in STEP 2, discards Copy 1 and distributes remaining copies as required.
Collector checks the Split or Single specimen collection box. If the collection is observed, Collector checks the Observed box and enters a remark in STEP 2.
STEP 3:
Donor watches Collector pour the specimen from the collection container into the specimen bottle(s), place the cap(s) on the specimen bottle(s), and affix the label(s)/seal(s) on the specimen bottle(s).
Collector dates the specimen bottle label(s)/seal(s) after placement on the specimen bottle(s).
Donor initials the specimen bottle label(s)/seal(s) after placement on the specimen bottle(s).
Collector turns to Copy 2 (Medical Review Officer Copy) and instructs Donor to read and complete the certification statement in STEP 5 (signature, printed name, date, phone numbers, and date of birth). If Donor refuses to sign the certification statement, Collector enters a remark in STEP 2 on Copy 1.
STEP 4:
Collector completes STEP 4 on Copy 1 (signature, printed name, date, time of collection, and name of delivery service), placesthe sealed specimen bottle(s) and Copy 1 of the CCF in a leak-proof plastic bag, seals the bag, prepares the specimen packagefor shipment, and distributes the remaining CCF copies as required.
Analyte Maximum Concentrations:
| Initial test analyte | Initial test cutoff concentration | Confirmatory test analyte | Confirmatory test cutoff concentration |
| Marijuana metabolites | 50 ng/mL | THCA1 | 15 ng/mL |
| Cocaine metabolites | 150 ng/mL | Benzoylecgonine | 100 ng/mL |
| Opiate metabolites Codeine/Morphine2 | 2000 ng/mL | Codeine Morphine | 2000 ng/mL |
| 6-Acetylmorphine | 10 ng/mL | 6-Acetylmorphine | 10 ng/mL |
| Phencyclidine | 25 ng/mL | Phencyclidine | 25 ng/mL |
| Amphetamines3 AMP/MAMP4 | 500 ng/mL | Amphetamine Methamphetamine5 | 250 ng/mL |
| MDMA6 | 500 ng/mL | MDMA MDA7 MDEA8 | 250 ng/mL |
1 Delta-9-tetrahydrocannabinol-9-carboxylic acid (THCA).
2 Morphine is the target analyte for codeine/morphine testing.
3 Either a single initial test kit or multiple initial test kits may be used provided the single test kit detects each target analyte independently at the specified cutoff.
4 Methamphetamine is the target analyte for amphetamine/methamphetamine testing.
5 To be reported as positive for methamphetamine, a specimen must also contain amphetamine at a concentration equal to or greater than 100 ng/mL.
6 Methylenedioxymethamphetamine (MDMA).
7 Methylenedioxyamphetamine (MDA).
8 Methylenedioxyethylamphetamine (MDEA).

